
A medical device known as transvaginal mesh can cause a variety of health problems that may require additional revision surgeries.
There is an little-known truth behind a medical device called transvaginal mesh. While it has many benefits, it is also associated with dangerous side effects.
Transvaginal mesh is a highly controversial medical device that has left thousands of women suffering. In many cases, the so-called permanent fix will require additional care and surgeries.
What is Transvaginal Mesh?

Prolapse occurs when parts of the body slip down or forward.
Image: Women’s Health Group
Transvaginal mesh is a polypropylene plastic implant that is surgically placed within the vagina. The plastic material is formed into various shapes to treat different medical issues. It can be classified as synthetic, biologic or a compound material.
The textured plastic creates lightweight pores that hinder inflammation in the body. The device is marketed as a permanent cure for pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Mesh is recommended for women who recently gave birth, had a hysterectomy or are undergoing menopause. For women with hysterectomies, the device can support a vagina that has dropped after the uterus was removed.
Pelvic organ prolapse is marked by an abnormal detachment of the bladder, rectum and uterus. The mesh catches the falling organs and holds them in place.
On the other hand, stress urinary incontinence is the inability to control the bladder. The mesh raises the urethra to a normal positioning. While mesh offers solutions to certain conditions, it can also create a slew of new issues.
Implantation Complications
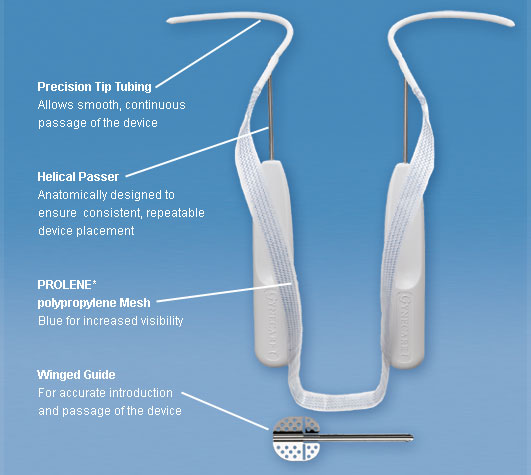
Transvaginal mesh often comes in a variety of different shapes and sizes to suit the needs and body type of each woman.
Image: Global Library of Women’s Medicine
Although the device has been on the market for decades, transvaginal mesh has been linked to a variety of complications. The FDA received more than 4,000 reports of complications between 2006 and 20011.
The implantation of transvaginal mesh can cause infections and problems with tissues and organs, in addition to a variety of other symptoms. The device may cause pain, nerve damage, bleeding, pain during intercourse, scarring and much more. The transvaginal mesh can deteriorate or migrate and cause additional issues, creating the need for a revision surgery.
The transvaginal mesh can become fused to surrounding organs and tissue. Because it is meant to be a permanent solution to medical problems, it often takes multiple surgeries to remove.
Mesh Lawsuits On The Rise

Martha Salazar was awarded $73 million in a 2014 transvaginal mesh lawsuit in Dallas.
Image: Dallas Observer
Transvaginal mesh was approved by the FDA in 1996 through the 510(k) process. Many critics claim the expedited approval process leaves little time for safety and effectiveness testing.
Almost 70,000 lawsuits are awaiting in West Virginia district courts alone, along with thousands in other state courts.
If your vaginal mesh failed and required additional surgery, then you might be entitled to compensation for receiving a faulty medical device.
Click here to complete a free, no-obligation case evaluation to see if you qualify.
CitizensReport
Latest posts by CitizensReport (see all)
- 4 Natural Alternatives To Energy Drinks That Will Make You Feel Healthier - February 19, 2018
- NBC Nightly News Publishes Expose On IVC Filters - February 18, 2018
- Good Sugar, Bad Sugar: Why You Should Eat More Fruit - February 16, 2018


Join the discussion